Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 , Zn
, Zn +, and Co
+, and Co
Kato, Tomoaki*; Yu, Q.*; Tanaka, Kazuya; Kozai, Naofumi; Saito, Takumi*; Onuki, Toshihiko
Journal of Environmental Sciences, 86, p.78 - 86, 2019/12
Times Cited Count:2 Percentile:8.42(Environmental Sciences)This paper investigated the fate of the dissolved permanganate in aqueous solution after contact with bacterial cells and metal accumulation during precipitation of Mn oxides. When Mn(VII) was contacted with bacterial cells, cells were damaged and Mn(VII) was reduced by cells to lower valence and precipitated as Mn oxides (biomass Mn oxides). When Co ions were present, Co was incorporated into Mn oxides as Co
ions were present, Co was incorporated into Mn oxides as Co . These results suggest that Mn(VII) can be used to remove metal ions when introduced to wastewater as disinfectant.
. These results suggest that Mn(VII) can be used to remove metal ions when introduced to wastewater as disinfectant.
Tokunaga, Kohei*; Takahashi, Yoshio*
Environmental Science & Technology, 51(16), p.9194 - 9201, 2017/08
Times Cited Count:45 Percentile:83.05(Engineering, Environmental)In the present study, we explore a new application of barite (BaSO ) as a sequestering phase for selenite (Se(IV)) and selenate (Se(VI)) ions from aqueous solutions due to the low solubility and high stability of barite. The uptake of Se(IV) and Se(VI) during coprecipitation with barite was investigated through batch experiments to understand the factors controlling effective removal of Se(IV) and Se(VI) from polluted water to barite. The uptake of Se(IV) by barite is dependent on pH, coexistent calcium ion, and sulfate concentration in the initial solution, possibly due to their effects on the chemical affinity and structural similarity. On the other hand, the uptake of Se(VI) by barite was strongly dependent on sulfate concentration in the initial solution, which is only related to the structural similarity. This study provides a good estimate of its ability to effectively remove Se(IV) and Se(VI) from aqueous solutions (more than 80%) under optimized experimental parameters.
) as a sequestering phase for selenite (Se(IV)) and selenate (Se(VI)) ions from aqueous solutions due to the low solubility and high stability of barite. The uptake of Se(IV) and Se(VI) during coprecipitation with barite was investigated through batch experiments to understand the factors controlling effective removal of Se(IV) and Se(VI) from polluted water to barite. The uptake of Se(IV) by barite is dependent on pH, coexistent calcium ion, and sulfate concentration in the initial solution, possibly due to their effects on the chemical affinity and structural similarity. On the other hand, the uptake of Se(VI) by barite was strongly dependent on sulfate concentration in the initial solution, which is only related to the structural similarity. This study provides a good estimate of its ability to effectively remove Se(IV) and Se(VI) from aqueous solutions (more than 80%) under optimized experimental parameters.
 -ray spectrometry; Application of
-ray spectrometry; Application of  pre-concentration from large volume of water sample using Powdex resin and barium sulfate coprecipitation of radium isotopes
pre-concentration from large volume of water sample using Powdex resin and barium sulfate coprecipitation of radium isotopesTomita, Jumpei; Abe, Takuya
JAEA-Research 2016-026, 12 Pages, 2017/03
An analytical method of low-level Ra isotopes in freshwater samples with combination of  pre-concentration from large volume of water sample (
pre-concentration from large volume of water sample ( 170 L) using Powdex resin and
170 L) using Powdex resin and  -ray spectrometry followed by simple coprecipitation of Ra was developed.
-ray spectrometry followed by simple coprecipitation of Ra was developed.  pre-concentration of Ra by batch method using Powder resin was examined, and it was shown that the amount of the resin required collecting Ra in the water sample could be determined by measuring the electric conductivity (EC) of water sample. It was found that coprecipitation of Ra with barium sulfate could remove more than 96% of potassium that increases the background. The validation of this method was confirmed by the analyses of 170 L of water sample containing the known amount of Ra isotopes with different EC. Among the analyses, the recovery of Ra was 98% in average and detection limits of
pre-concentration of Ra by batch method using Powder resin was examined, and it was shown that the amount of the resin required collecting Ra in the water sample could be determined by measuring the electric conductivity (EC) of water sample. It was found that coprecipitation of Ra with barium sulfate could remove more than 96% of potassium that increases the background. The validation of this method was confirmed by the analyses of 170 L of water sample containing the known amount of Ra isotopes with different EC. Among the analyses, the recovery of Ra was 98% in average and detection limits of  Ra and
Ra and 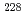 Ra were achieved to be approximately 0.3 and 0.5 mBq L
Ra were achieved to be approximately 0.3 and 0.5 mBq L , respectively.
, respectively.
Watanabe, Satoshi; Ishioka, Noriko; Osa, Akihiko; Koizumi, Mitsuo; Sekine, Toshiaki; Kiyomiya, Shoichiro*; Nakanishi, Hiromi*; Mori, Satoshi*
Radiochimica Acta, 89(11-12), p.853 - 858, 2002/02
Times Cited Count:17 Percentile:74.85(Chemistry, Inorganic & Nuclear)no abstracts in English
; Murata, Eiichi*; Sawahata, Yoshikazu*; Saito, Akira*
JNC TN8430 2001-002, 43 Pages, 2001/02
Japan Nuclear Cycle Development Institute (JNC) is designing the Low level radioactive Waste Treatment Facility (LWTF) in the Tokai Reprocessing Plant (TRP). The low level liquid waste generated the TRP is separated salt (NaNO , etc) and radionuclide in liquid treatment process of LWTF. The process can get higher volume reduction than previous bituminization. Based on the engineering tests equal to the liquid treatment process of LWTF, the validity of operational condition in LWTF is evaluated. As the results, it is confirmed that all operational condition in the processes which is Iodine immobilization, Pre-filter filtration, Pre-treatment, Coprecipitation and Ultrafiltration are available.
, etc) and radionuclide in liquid treatment process of LWTF. The process can get higher volume reduction than previous bituminization. Based on the engineering tests equal to the liquid treatment process of LWTF, the validity of operational condition in LWTF is evaluated. As the results, it is confirmed that all operational condition in the processes which is Iodine immobilization, Pre-filter filtration, Pre-treatment, Coprecipitation and Ultrafiltration are available.
Nagano, Tetsushi; Mitamura, Hisayoshi; Nakayama, Shinichi; Nakashima, Satoru*
Clays and Clay Minerals, 47(6), p.748 - 754, 1999/00
Times Cited Count:16 Percentile:44.62(Chemistry, Physical)no abstracts in English
Uchiyama, Gunzo; Maeda, Mitsuru; Fujine, Sachio; *
ICEM 95: Proc. of 5th Int. Conf. on Radioactive Waste Management and Environmental Remediation,Vol. 1, 0, p.403 - 407, 1995/00
no abstracts in English
Sumiya, Shuichi; Hayashi, Naomi; ; Narita, Osamu
PNC TN8430 91-001, 45 Pages, 1990/12
A radioanalytical method for low level samarium-151(Sm-151) and promethium-147(Pm-147) in environmental samples has been studied for the environmental assessment around nuclear facilities. In this study, we use the separation method with high performance liquid chromatography (HPLC) to determine Sm-151 and Pm-147 in environmental samples such as sea sediments and marine organisms. Samarium-151 and Pm-147 in environmental samples are coprecipitated with other lanthanoids after adding neodymium(Nd). These nuclides are purified by anion exchange methods in methanol-mineral acid media. After the purification, Sm-151 and Pm-147 are separated with HPLC in lactic acid-sodium hydroxide media, and determined with liquid scintillation counting, respectively. The Nd is determined by inductively coupled plasma atomic emission spectrometry (ICP-AES) to correct chemical recoveries of these nuclides. The detection limits for Sm-151 and Pm-147 in this method are about 0.01Bq/sample.
Kimura, Takaumi
Journal of Radioanalytical and Nuclear Chemistry, 139(2), p.307 - 314, 1990/00
Times Cited Count:2 Percentile:31.34(Chemistry, Analytical)no abstracts in English
Kimura, Takaumi
Journal of Radioanalytical and Nuclear Chemistry, 139(2), p.297 - 305, 1990/00
Times Cited Count:13 Percentile:76.1(Chemistry, Analytical)no abstracts in English
; ;
Radiochimica Acta, 39, p.179 - 183, 1986/00
no abstracts in English
;
Journal of Radioanalytical and Nuclear Chemistry, 94(6), p.381 - 390, 1985/00
Times Cited Count:3 Percentile:47.07(Chemistry, Analytical)no abstracts in English
;
Journal of Radioanalytical and Nuclear Chemistry, 91(1), p.59 - 65, 1985/00
Times Cited Count:15 Percentile:84.25(Chemistry, Analytical)no abstracts in English
; ; ; ;
Sep.Sci.Technol., 18(2), p.177 - 186, 1983/00
Times Cited Count:1 Percentile:33.51(Chemistry, Multidisciplinary)no abstracts in English
; ;
Journal of Nuclear Science and Technology, 13(10), p.591 - 595, 1976/10
Times Cited Count:8no abstracts in English
Radiochem.Radioanal.Lett., 19(1), p.33 - 42, 1974/01
no abstracts in English
Sato, Junya; Suzuki, Shinji; Nakagawa, Akinori; Kato, Jun; Sakakibara, Tetsuro; Nakazawa, Osamu; Yamashita, Masaaki; Sato, Fuminori; Sukegawa, Hirobumi; Meguro, Yoshihiro
no journal, ,
no abstracts in English
 into Mn oxides produced by MnO
into Mn oxides produced by MnO
 reduction using biomass
reduction using biomassKato, Tomoaki; Onuki, Toshihiko; Yu, Q.
no journal, ,
Various radionuclides were released into seawater by the accident of the Fukushima Daiichi Nuclear Power Plant. We need to establish techniques to eliminate such radionuclides from the contaminated seawater. Mn oxides are known to sorb various metal ions. Mn oxides can be produced by reduction of MnO
 using biomass (biomass-MnOX) although there is little information on metal uptake mechanisms during the formation of biomass-MnOX. In this research, we investigated the uptake of Sr
using biomass (biomass-MnOX) although there is little information on metal uptake mechanisms during the formation of biomass-MnOX. In this research, we investigated the uptake of Sr during the formation of biomass-MnOX produced by reduction of KMnO
during the formation of biomass-MnOX produced by reduction of KMnO with cells of Pseudomonas fluorescens. After inoculating the cells into the Mn(VII) and Sr
with cells of Pseudomonas fluorescens. After inoculating the cells into the Mn(VII) and Sr containing solution, blackish precipitates were formed in the bottom. EXAFS analysis of Sr
containing solution, blackish precipitates were formed in the bottom. EXAFS analysis of Sr in the precipitates showed that the presence of Mn around Sr, indicating that Sr was formed inner-sphere complex with Mn during coprecipitation.
in the precipitates showed that the presence of Mn around Sr, indicating that Sr was formed inner-sphere complex with Mn during coprecipitation.
Taniguchi, Takumi; Irisawa, Keita; Ito, Yuzuru; Namiki, Masahiro; Osugi, Takeshi; Abe, Tomohisa; Sato, Junya; Sakakibara, Tetsuro; Nakazawa, Osamu; Meguro, Yoshihiro; et al.
no journal, ,
no abstracts in English